Incidence of adverse events of the Covid-19 vaccine in a population of kidney transplant recipients
Verónica Gimeno-Hernán1,2, Belén Peix-Jiménez1, Isabel Pérez-Flores3, Arrianne Aiffil-Meneses4, Ismael Ortuño-Soriano2,3, Ana Sánchez-Fructuoso3
1Hospital Clínico San Carlos. Instituto de Investigación Sanitaria San Carlos (IdISSC). Madrid. Spain
2 Facultad de Enfermería, Fisioterapia y Podología. Universidad Complutense de Madrid. Instituto de Investigación Sanitaria San Carlos (IdISSC). Madrid. Spain
3 Hospital Clínico San Carlos. Facultad de Medicina. Universidad Complutense de Madrid. Instituto de Investigación Sanitaria San Carlos (IdISSC). Madrid. Spain
4 Hospital Clínico San Carlos. Madrid. Spain
https://doi.org/10.37551/S2254-28842024013
Como citar este artículo:
Gimeno-Hernán V, Peix-Jiménez B, Pérez-Flores I, Aiffil-Meneses A, Ortuño-Soriano I, Sánchez-Fructuoso A.
Incidence of adverse events of the Covid-19 vaccine in a population of kidney transplant recipients.
Enferm Nefrol. 2024;27(2):119-25
Correspondencia:
|
Recepción: 21-02-24
|
ABSTRACT
Introducción: Early published series suggest that most renal transplant recipients remain at high risk of SARS-CoV-2 infection due to poor humoral response after vaccination. The aim was to study the occurrence of adverse events after two doses of mRNA-1273 vaccine in a population of renal transplant recipients.
Material and Method: Analytical, observational, and prospective study. Subjects were injected with two doses of mRNA-1273 vaccine against SARS-CoV-2 according to the schedule established by the laboratory. After injection of each dose, and up to 72 hours later, participants recorded local and/or systemic symptoms and their intensity.
Results: 187 patients were included. Eighteen percent of them became infected with SARS-CoV-2 in the pre-vaccination period or between the 1st and 2nd dose. The incidence of adverse events was 91.2%. Of these, the incidence of local (62%) was higher than that of systemic (55%). Past infection was a risk factor for the occurrence of local adverse events after vaccination (OR= 2.4; p=0.045). The same association was detected for systemic adverse events, which were more frequent among those who had passed the disease (OR=3.83; p=0.003).
Conclusions: The mRNA-1273 vaccine does not appear to cause serious side effects. The incidence of local and systemic adverse events was higher in those patients with past disease.
Keywords: renal transplantation; mRNA-1273 vaccine; SARS-CoV-2 adverse reactions.
RESUMEN
Incidencia de eventos adversos de la vacuna frente a SARS CoV-2 en una población de receptores de trasplante renal
Introducción: Las primeras series publicadas sugieren que la mayoría de los receptores de trasplante renal siguen teniendo un alto riesgo de infección por SARS-CoV-2 debido a una pobre respuesta humoral tras la vacunación. El objetivo fue estudiar la presencia de eventos adversos tras 2 dosis de vacuna mRNA-1273 en una población de trasplantados renales.
Material y Método: Estudio analítico, observacional y prospectivo. A los sujetos se les inyectó dos dosis de la vacuna mRNA-1273 frente al SARS-CoV-2 según pauta establecida por el laboratorio. Tras la inyección de cada una de las dosis, y hasta las 72 horas posteriores, los participantes llevaron a cabo un registro de síntomas locales y/o sistémicos y la intensidad de los mismos.
Resultados: Se incluyeron 187 pacientes. Un 18% de ellos se infectaron de SARS-CoV-2 en el periodo prevacunación o entre la 1ª y 2ª dosis. La incidencia de eventos adversos fue de 91,2%. De ellos, la incidencia de los locales (62%) fue mayor que la de sistémicos (55%). Haber pasado la infección fue un factor de riesgo de aparición de eventos adversos locales tras la vacunación (OR=2,4; p=0,045). La misma asociación fue detectada en el caso de eventos adversos sistémicos, que fueron más frecuentes entre los que habían pasado la enfermedad (OR=3,83; p=0,003).
Conclusiones: La vacuna de mRNA-1273 no parece provocar efectos secundarios graves. La incidencia de eventos adversos locales y sistémicos fue mayor en aquellos pacientes que habían pasado la enfermedad.
Palabras clave: trasplante renal; vacuna mRNA-1273; SARS-CoV-2 reacciones adversas.
INTRODUCTION
The SARS-CoV-2 virus emerged in the city of Wuhan, China back in December 2019 and spread creating a global health crisis with vaccination being the only hope to control the pandemic1. Despite being an unknown virus until its appearance, vaccines with almost 100% efficacy have been developed to prevent moderate and severe disease in 90% of the overall population2.
In patients with kidney disease, all-cause infection remains one of the most common causes of morbidity and mortality. Changes in the immune system explain the increased risk associated with this population3 .
Solid organ transplant recipients have been excluded from vaccine clinical trials on purpose. It is likely that the immune response could be significantly compromised by their underlying primary comorbid conditions and immunosuppressive treatments received4. Scientific medical societies have recommended vaccination against SARS-CoV-2 for these patients, and the studies published to this date have found evidence that a significant number of kidney transplant recipients still has a high risk of SARS-CoV-2 infection and moderate-to-severe disease due to a deficient humoral response2.
The safety of vaccines is well established with a solid base of scientific evidence from clinical trials conducted prior to their commercialization and quality controls5,6. However, like any medication, vaccines can have unintended and harmful adverse effects that can occur coincidentally with the compatible temporal sequence following vaccine administration. Establishing causality in the occurrence of local adverse reactions is often straightforward. However, this is not always the case with systemic reactions7,8.
Therefore, a concern that has particularly worried transplant teams is the possibility that these new mRNA vaccines could alter graft functionality or cause severe side effects. Some authors have described that symptoms following vaccine administration in this population are similar to those of the overall population. Also, no safety issues contraindicating their administration have been identified9. However, information is still limited, and therefore, studying systemic and local reactions due to SARS-CoV-2 vaccination in transplant patients will help increase confidence in vaccination for both the healthcare personnel and patients who still remain hesitant to be vaccinated.
Therefore, the objective of this research was to study the safety of mRNA-1273 vaccine (Moderna) against SARS-CoV-2, evaluate the presence of adverse effects and the possible impact on renal function.
MATERIAL AND METHOD
Study Design and Participants
This was an analytical, observational, and prospective cohort study conducted in a tertiary hospital of the Community of Madrid, Spain.
The study was proposed to 211 patients with a functioning kidney transplant who received the SARS-CoV-2 vaccine between March 19th and April 24th, 2021. Ultimately, a total of 187 individuals who agreed to participate and undergo post-vaccination follow-up were included. Patients with active tumors and those experiencing SARS-CoV-2-like symptoms at the time of vaccination were excluded.
Study procedure
The subjects received 2 doses of the mRNA-1273 vaccine against SARS-CoV-2 following the pattern established by the laboratory that consisted of an injection on day 0 and a second dose 28 days later. The center sent a text message to all transplant recipients indicating the date and time for vaccination. Researchers, by the way, were not responsible for the selection process.
Following each dose, and up to 72 hours afterward, participants recorded symptoms, both local and systemic, on a questionnaire specifically designed for this purpose. After obtaining informed consent, the responsible nephrologist provided the patient with the registration form, and the nurse at the Renal Transplant Clinic collected the results during the patient’s next visit. For each adverse event (AE), the onset time and intensity were recorded using a numerical scale from 1 to 3 being, 1 indicative of the lowest intensity and 3 the more. The local AEs recorded included pain, redness, swelling, and itching all at the injection site, axillary lymphadenopathy or swelling of neck/clavicle lymph nodes. Systemic AEs were classified as fever or low-grade fever, headache, fatigue, digestive symptoms, myalgia, chills, general malaise, and dizziness or hypotension, as well as other unexpected symptoms.
Regarding the analysis of results, a database was created including the AEs and the following variables: comorbidities (like hypertension, diabetes), sociodemographic factors (age, sex), transplant-related factors (years since kidney transplantation, immunosuppressive treatment received, glomerular filtration rate measured by CKD-EPI prior to vaccination), and vaccination-related factors (prior SARS-CoV-2 infection before or during the vaccination process, positive humoral immune response defined as IgG titers in blood ≥ 50 AU/mL, and positive cellular response, CD4 and/or CD8).
Statistical Analysis
Quantitative variables were expressed as mean and standard deviation if they followed a normal distribution or with as median and interquartile range in cases of skewed distribution. Qualitative variables were expressed using percentages and total values. For the analysis of quantitative variables, the Student t test was used, and for the analysis of qualitative variables, the chi-square test was used. Finally, logistic regression was used for multivariate analysis to examine the relationship between each study variable and the occurrence of adverse events. The statistical analysis of results was performed using SPSS 26.0 statistical software package for Windows.
Ethical Considerations
Participation in the study was voluntary, and participants could withdraw their consent and leave the study at any time without this having any impact on their medical care relationship.
The project was conducted based on the latest version available of the Helsinki Declaration and the Good Clinical Practice Guidelines of the International Conference on Harmonization (GCP/ICH).
All personal data were identified with a code, and only the researcher could associate the data available with the patient and their medical history.
The data described in this study are part of a protocol that has been approved by Hospital Clínico San Carlos Ethics Committee.
RESULTS
The patients included had a mean age of 58.3 ± 13.4 years (61.2% were man; n=114). The median time elapsed since transplantation was 9.6 years (IQR, 5.07-15). Among them, 18% (n=34) were infected with SARS-CoV-2 in the pre-vaccination period. A total of 92.8% (n=178) of the patients had hypertension, and 28.9% (n=54) diabetes. The mean glomerular filtration rate measured by CKD-EPI before and after vaccination was 50.78 (±21.48) mL/min/1.73 m² and 53.33 (±27.75) mL/min/1.73 m², respectively (P=0.98).
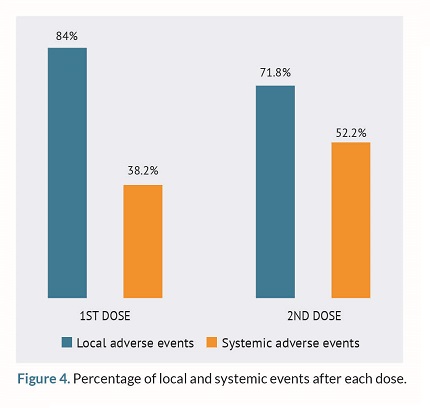
The overall rate of adverse events was 91.2% (n = 165) with local events (n=151; 80.8%) being more common compared to systemic events (n=114; 60.8%).
Among the reported local AEs, the most common was local pain at the injection site (n=119; 64%) followed by swelling (n=49; 26.43%) and redness in the region of interest (n=26; 14.44%). The frequency of pain and erythema increased from the first to the second dose as shown in figure 1 while itching, swelling, and lymphadenopathy decreased as seen in figure 1.
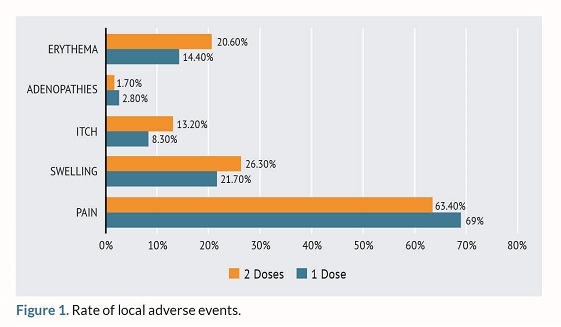
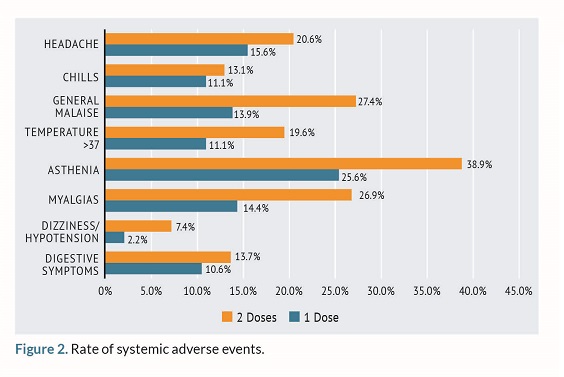
Among the systemic AEs, the most commonly reported ones were asthenia (n=73; 39.06%) and myalgia (n=50; 27.1%). Systemic AEs were more frequent after the administration of the second dose compared to the first one as seen on figure 2.
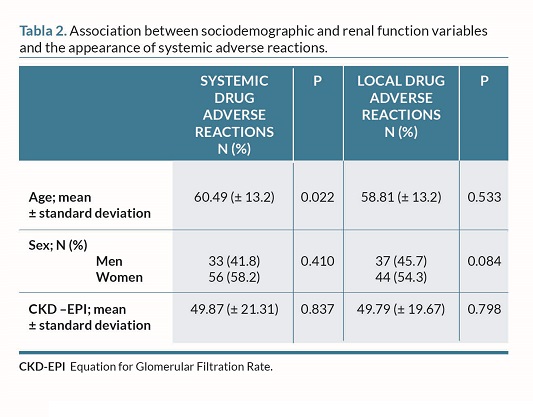
In the univariate analysis, a significant difference was seen based on age such that a higher number of years was associated with a lower number of systemic reactions in the population studied (P=0.022).
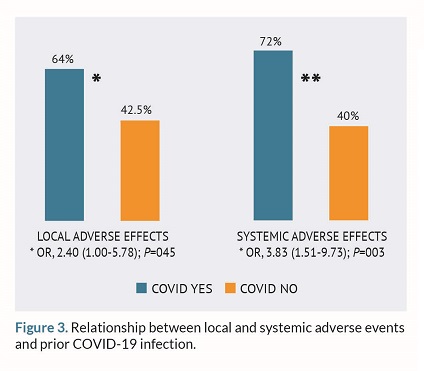
Also, an association was also found between the rate of AEs and prior infection with SARS-CoV-2: the percentage of local effects, in general, was higher among those who had a previous infection (n=120; 64%) compared to those who had not been infected at all (n=88; 47.5%). Therefore, prior infection was a risk factor for the occurrence of local AEs after vaccination (OR, 2.4; 95%CI, 1.00-5.78; P=0.045). The same association was seen for systemic AEs, which were more common among those who had previously had the disease (OR, 3.83; 95%CI, 1.51-9.7; P=0.003).
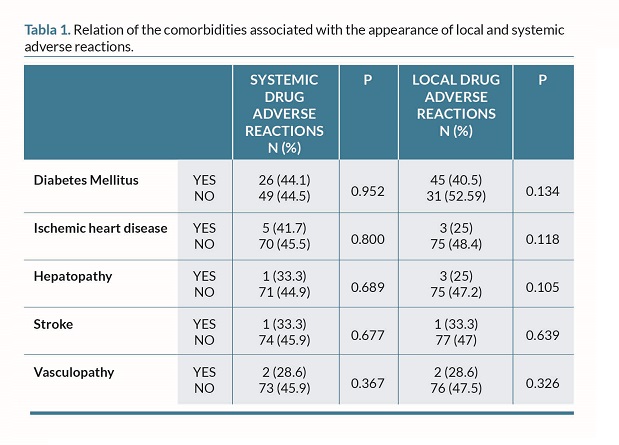
In the multivariate analysis, prior COVID infection remained significantly associated with systemic AEs (OR, 3.42; 95%CI, 1.33-8.77; P=0.011), while age approached the significance threshold (OR, 0.97; 95%CI, 0.95-1.00; P=0.051) (table 1 and 2).
Following the administration of both doses, local AEs were more common compared to the systemic ones as shown on figure 4.
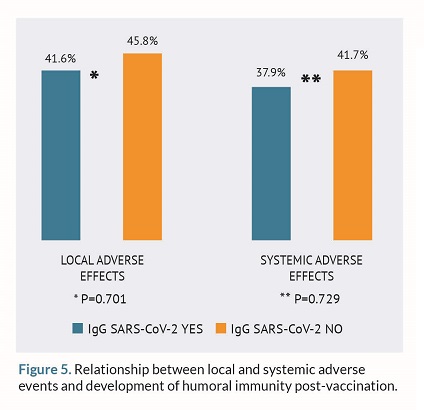
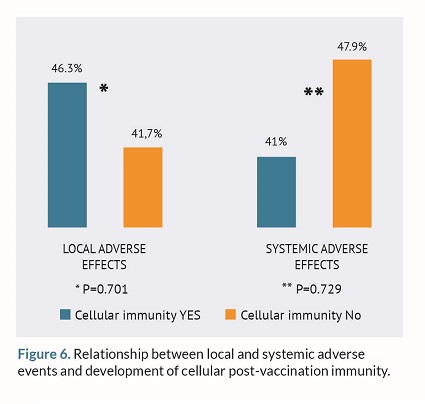
Finally, the relationship between local and systemic AEs with the development of humoral and cellular immunity post-vaccination was studied. However, no statistically significant differences were found as shown in figures 5 and 6.
DISCUSSION
The unique circumstances surrounding the renal transplant process like associated comorbidities and immunosuppression put transplant recipients at a higher risk for developing severe disease from SARS-CoV-210. Therefore, effective and safe vaccination is considered crucial in this population.
Back in 2021, Spanish health authorities recommended a three-dose regimen of mRNA vaccines against SARS-CoV-2 for immunocompromised individuals assuming a lower immunogenicity to these vaccines in solid organ transplant recipients compared to the overall population12.
Regarding the sociodemographic variables of the study sample, they are consistent with those of former studies conducted on renal transplant recipients in the Spanish population13. This suggests that the findings associated with adverse reactions to the vaccine in this study could be extrapolated to the renal transplant population.
The data from this research show an association between age and the number of systemic adverse reactions reported. This finding is consistent with the data reported for the overall population in the pharmacovigilance report issued by the Spanish Agency of Medicines and Medical Devices (AEMPS)14 regarding COVID-19 vaccines. It indicates that overall adverse reactions were reportedly 10 times more common in individuals under 65 years of age and 3 times more common in women. These differences may be attributed to immune, genetic, and hormonal factors15, although no gender differences were found in our study.
Regarding immunosuppressive treatment of transplant recipients included in the study, the data are very much consistent with those reported in the study conducted by López-Oliva et al16. The approaches followed for cases of SARS-CoV-2 infection were similar among the different Spanish hospitals involved since with mild symptoms treatment was not warranted. However, as the infection grew adjustments were made based on the patient’s needs.
During the vaccination process against SARS-CoV-2, it has been reported that mRNA vaccines can cause mild to moderate side effects with local adverse events (AE) being predominant. The most common local AE reported is pain at the injection site, which is consistent with the findings of this research13.
The rate of AEs in the study population increased from the first to the second dose as observed in the investigation conducted by Massa et al13. However, other studies have reported that reactogenicity with booster doses was higher especially regarding systemic adverse effects17. This is consistent with the results found in our study as shown in figure 2.
The findings of our study are similar to those reported in clinical trials of the BNT1162b and mRNA-1273 vaccines being pain at the injection site and asthenia two of the most commonly reported symptoms in healthy adults just like in our study population18.
Overall, the adverse reactions reported by the patients in this study are similar to those reported in the general population by the AEMPS14. However, there is a discrepancy in the percentages of symptom presentation being systemic AEs (headache [21%], fever [33%], and myalgia [15%]) more prevalent in the overall population and local AEs (pain at the injection site reported by 13% of the sample) more prevalent in renal transplant recipients. The reason for this difference may be attributed to the method of information collection, as our study methodology relied on a questionnaire while the data reported by the AEMPS come from pharmacovigilance changes that may not be as comprehensive.
In this study, no significant safety issues like myocarditis or neurological symptoms were observed, which is somehow similar to what has been reported in clinical trials and vaccine registries of mRNA-based COVID-19 vaccines19,20.
In the study conducted by Connolly et al.21, patients on immunosuppressive therapies for rheumatic and musculoskeletal diseases who received the BNT162b2 or mRNA-1273 vaccine reported that 69% of participants experienced, at least, one systemic side effect being fatigue the most commonly reported symptom, and 86% reporting local symptoms being pain at the injection site the most prevalent of all. These results are consistent in terms of percentages (62% for local AEs and 55% for systemic AEs) and symptomatology with those found in our study. This coincidence may be due to both studies being conducted in immunocompromised patients. However, these data were only collected after the first vaccine dose and were obtained through a questionnaire from those who voluntarily decided to participate, which could introduce information bias.
In the study conducted by Fernández Prada M.22, that focused on the safety and efficacy profile of other vaccines administered to immunocompromised patients, the rate of AEs was higher with inactivated virus vaccines at around 88.7%. Regarding the percentage of adverse event reports, 35.8% were local, 49.1% systemic, and 15.1% both types. The difference with the results found in our study could be attributed to the type of vaccine and the specific dosing regimen administered to the patients. However, the symptoms reported by the patients were similar being pain and fever the most commonly described symptoms of all22.
The main strengths of our study include a representative sample of the renal transplant population as participation was offered to all vaccinated patients. Also, the rate of refusal was low. The information was collected thoroughly using a specifically designed questionnaire. However, a major limitation is that it is a single-center study.
CONCLUSIONS
The conclusions drawn from the results obtained in this study are of great relevance to the medical community, as they provide crucial data on the frequency of adverse events caused by the SARS-CoV-2 vaccine in kidney transplant patients. Systemic and local adverse events were found to be significantly more frequent in subjects who had previously been infected with SARS-CoV-2. Additionally, the rate of systemic adverse events was higher in younger patients, showing a significant association. In our sample, vaccination mostly resulted in local adverse events, which is consistent with the overall population.
BIBLIOGRAFÍA
1. Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated 315 with a new coronavirus of probable bat origin. Nature. 2020; 579(7798):270-3.
2. Martín De Francisco A. Vacunas Sars-Cov 2. Nefrología. 2021 [cited 16 May 2022]. Available at: https://nefrologiaaldia.org./es-articulo-vacunas-saars-cov2-marzo-2021-366
3. Dinits-Pensy M, Forrest GN, Cross AS, Hise MK. The use of vaccines in adult patient with renal disease. Am J Kidney Dis. 2005;46(6):997-1011.
4. Haddadin Z, Krueger K, Thomas LD, Overton ET, Ison M, Halasa N. Alternative strategies of posttransplant influenza vaccination in adult solid organ transplant recipient. Am J transplant. 2021;21:938-49.
5. Cunningham AL, Garçon N, Leo O, Friedland LR, Strugnell R, Laupèze B, et al. Vaccine development: from concept to early testing. Vaccine.2016;34(52):6655-64.
6. Comité Asesor de Vacunas (CAV-AEP). Madrid: Manuel Merino Moína; [cited 19 feb 2023]. Manual de Vacunas en línea de la AEP; Capítulo 3. Seguridad de las vacunas. Contraindicaciones y precauciones. Available at: http://vacunasaep.org/ documentos/manual/cap-37
7. Escudero C, García-Fernández C, Ibáñez MD. Reacciones alérgicas a las vacunas. Vacunas. 2008;9:156-60.
8. Eseverri JL, Ranea S, Marin, A. Reacciones adversas a vacunas. Allergol Immunopathol. 2003;31:125-38.
9. Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. Engl J Med. 2021;384:403-16.
10. Cravedi P, Mothi SS, Azzi Y, Haverly M, Farouk SS, Pérez-Sáez MJ, et al. COVID-19 and kidney transplantation: Results from the TANGO International Transplant Consortium. Soy J Trasplante. 2020;20(11):3140-8.
11. Azzi Y, Parides M, Alani O, Loarte-Campos P, Bartash R, Forest S, et al. COVID-19 infection in kidney transplant recipients at the epicenter of pandemics. Kidney Int. 2020;98(6):1559–67.
12. Dirección General de Salud Pública. Consejería de Sanidad. Documento técnico de vacunación frente a COVID-19 en la Comunidad de Madrid. Comunidad de Madrid; 2020 [actualized 2022; cited 5 May 2022]. Available at: https://www.comunidad.madrid/servicios/salud/vacunacion-frente-coronavirus-comunidad-madrid
13. Masa F, Grupper A, Rabinowich L, Schwartz D, Schwartz IF, Ben-Yehoyada M, Shashar M, et al. Reduced humoral response to mRNA SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients without prior exposure to the virus. Soy J Trasplante. 2021;21:2719-26.
14. Agencia Española del Medicamento y Producto Sanitario. 19º Informe de Farmacovigilancia sobre vacunas COVID-19. Madrid. 2023 (Actualización 2023) [consultado 10 Feb 2022]. Available at: https://www.aemps.gob.es/informa/19o-informe-de-farmacovigilancia-sobre-vacunascovid-19/#index3.1
15. Comité Asesor de Vacunas. Madrid: Manuel Merino Molina; [consultado 23 Feb 2023]. Manual de vacunas en línea de la AEP; Capítulo 2. Coadmnistración de las vacunas entre sí y con otros productos biológicos. Available at: https://vacunasaep.org/documentos/manual/cap-2
16. López-Oliva MO, Pérez-Flores I, Molina M, José Aladrén M, Trujillo H, Redondo-Pachón D, et al. Manejo de la inmunosupresión en pacientes trasplantados de riñón con COVID19. Estudio multicéntrico nacional derivado del registro COVID de la S.E.N. Nefrologia. 2022.1053:1-10.
17. Reindl-Schwaighofer R, Heinzel A, Mayrdorfer M, Jabbour R, Hofbauer TM, Merrelaar A, et al. Comparison of SARS-CoV-2 Antibody Response 4 Weeks After Homologous vs Heterologous Third Vaccine Dose in Kidney Transplant Recipients: A Randomized Clinical Trial. JAMA Intern Med. 2022;182(2):165-71.
18. Grupper A, Rabinowich L, Schwartz D, Schwartz IF, Ben-Yehoyada M, Shashar M, et al. Reduced humoral response to mRNA SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients without prior exposure to the virus. Am J Transplant. 2021;21(8):2719-26.
19. Polack FP, Thomas SJ, Kitchin N, et al. for the C4591001 Clinical Trial Group. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603-15.
20. Baden LR, El Sahly HM, Essink B, et al. for the COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403-16.
21. Damiani G, Allocco F, Network YD, Malagoli P. COVID-19 vaccination and patients with psoriasis under biologics: Real-life evidence on safety and effectiveness from Italian vaccinated healthcare workers. Clin Exp Dermatol. 2021; 46(6):1106-8
22. Fernández-Prada M, Viejo-González A, Martínez-Torrón A, Martínez-Ortega C, Ruiz-Salazar J, Huerta-González I. Reacciones adversas asociadas a la vacunación en pacientes inmunodeprimidos y en situaciones especiales de una Unidad de Vacunas hospitalaria. Rev Esp Quimioter. 2019;32(5):432-9.
Este artículo se distribuye bajo una Licencia Creative Commons Atribución–NoComercial 4.0 Internacional.
https://creativecommons.org/licenses/by-nc/4.0/
